Learn How To Measure COVID-19 Vaccine’s Effectiveness!
In order to understand the usefulness of the MaxNAb Kit ELISA for NEUTRALIZING ANTIBODIES to SARS-CoV-2 in measuring the effectiveness of vaccination and its difference with other antibody screening tests against this virus, we must study some aspects of the immune response to COVID-19, the serological antibody tests used for diagnosis, and the fundamentals of vaccines against COVID-19.
Clinical differences between children and adults
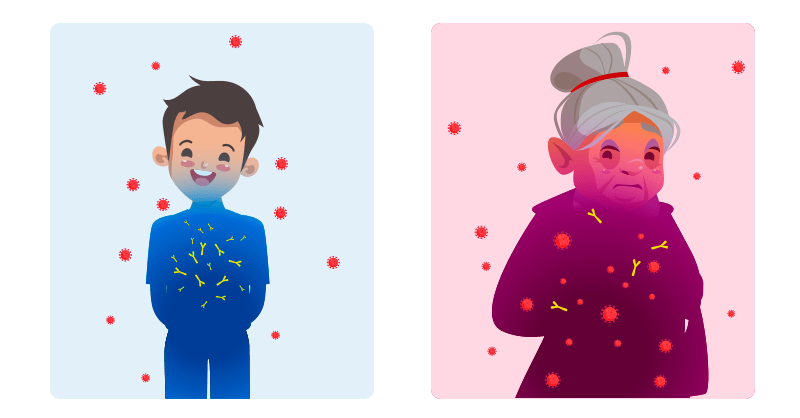
Most children infected with SARS-CoV-2 are asymptomatic or have mild symptoms: cough, fever, or gastrointestinal symptoms.
Infants younger than 1 year and children with certain underlying medical conditions have an increased risk of developing a severe form of COVID-19.
In some rare cases, children who develop COVID-19 have a medical condition called “Multisystem inflammatory syndrome in children” (MIS-C). This condition causes inflammation of the eyes, skin, brain, kidneys, lungs, heart, or gastrointestinal tract, which sometimes begins weeks after they have contracted SARS-CoV-2.
Adults are more likely than children to develop a serious or critical illness from COVID-19, especially those who are older or those with certain underlying medical conditions, such as obesity, type 2 diabetes, chronic kidney disease, and cancer.
Severe illness often requires hospitalization and may include management of complications such as “Acute respiratory distress syndrome” (ARDS), pneumonia, blood clots, acute kidney injury, sepsis, or cardiomyopathy.
A study by researchers at Columbia University Irving Medical Center in New York (CUIMC) in November 2020 showed clear differences between the antibody responses of children and adults with COVID-19. In particular, differences were observed in the type, amount, and neutralizing activity of antibodies.
Different antibody responses
People with a SARS-CoV-2 infection and those who have recovered from mild illness show specific antibodies against SARS-CoV-2 coronavirus proteins: spike (S) proteins and nucleocapsid proteins (N).
The virus’ S protein binds to the cell’s receptor, allowing the virus to enter. The N protein is required for SARS-CoV-2 replication.
Anti-S antibodies can effectively neutralize viral activity.
In the research, adults generated anti-S antibodies: immunoglobulin G (IgG), immunoglobulin M (IgM), and immunoglobulin A (IgA). There were significantly higher concentrations of these antibodies in the COVID-ARDS group.
In contrast, in children and adult convalescent plasma donors (CPD), there was a similar pattern of anti-S antibodies: predominance of IgG and low levels of anti-S IgM.
The level of anti-N IgG antibodies was significantly lower in children than in adults and had less neutralizing activity.
The group of adults with COVID-ARDS had a higher concentration and variety of anti-SARS-CoV-2 antibodies and higher neutralizing activity.
These results suggest that anti-N antibody concentrations depend on age rather than symptoms.
Reason for the differences

“This is a new infection for everyone, but children are uniquely adapted to see pathogens for the first time. That’s what your immune system is designed for. Children have many naive T cells that can recognize all kinds of new pathogens, while older people rely more on immune memory. We are not as capable of responding to a new pathogen as children,” explains study co-director Donna Farber, Ph.D.
Because children’s immune systems successfully eliminate the virus, this could result in a reduced functional antibody response compared to adults.
The milder course of the disease in children is also compatible with lower concentrations of anti-N antibodies. This is because N proteins are only released after the destruction of SARS-CoV-2-infected cells.
According to Farber, “There is a connection between the magnitude of the immune response and the magnitude of the infection: the more severe the infection, the more robust the immune response because you need to have more cells and immune reactions to eliminate a higher dose of a pathogen.”
Children can also generate a more intense innate immune response to SARS-CoV-2 than adults. The innate immune response causes the release of interferon, which interferes with viral replication, and macrophages, which engulf and digest viruses.
Farber adds, “If the innate response is really strong, that can reduce the viral load in the lungs, and the antibodies and T cells of the adaptive response have less to eliminate.”
In addition, children may produce fewer angiotensin-converting enzyme 2 (ACE2) receptors in the cells lining their airways than adults. ACE2 is a protein on the surface of human cells through which SARS-CoV-2 enters cells.
ANTIBODY TESTS FOR COVID-19
After patients recover from COVID-19 antibodies can be detected in the blood for several months or more.
There is currently not enough evidence to know how long these antibodies last in the blood or whether infection with the virus in the past protects against a new infection. In fact, there are some known confirmed cases of reinfection.
Antibody tests detect certain types of antibodies related to COVID-19:
- Binding antibodies.
Rapid serological tests detect whether antibodies have been developed in response to a SARS-CoV-2 infection, but do not indicate how effective or extensive the immune response is.
- Neutralizing antibodies.
Newer, more sensitive test, which detects a subset of antibodies that inactivate the virus to determine how effective those antibodies are in blocking the SARS-CoV-2 virus to help protect against a new infection.
The rapid serological test detects the presence of antibodies generated in response to the infection of the IgG, IgM, and IgA isotypes against virus-specific proteins such as those of the spicules (S) and nucleocapsid (N), in serum and/or plasma samples from patients.
After the first week of infection, IgM antibodies begin to be detectable in the blood and remain for 2 to 3 weeks, while, usually after the second week, IgG antibodies appear that last over time.
These tests are used as a tool to increase the diagnostic capacity of COVID-19, in conjunction with molecular tests based on the reverse transcriptase-polymerase chain reaction (RT-PCR) technique that detects the viral genome, which can yield negative results in the late stages of infection.
On the other hand, it should be noted that a negative antibody result does not rule out infection with the virus, especially in the early stages when these antibodies against the virus are not yet detectable.
NEW COVID-19 ANTIBODY TEST TO REFLECT IMMUNITY TO THE SARS-COV-2 VIRUS MORE ACCURATELY
Existing tests detect whether a person’s blood contains antibodies that bind to the entire virus, a protein in their capsid, or the spicule protein that gives SARS-CoV-2 its characteristic crown-shaped structure.
According to a team of researchers, many from the University of North Carolina (UNC), (June 2020), there is a high probability that some people with antibodies to other coronaviruses will receive false-positive results from such tests.
This is because the various coronaviruses have many characteristics in common, particularly some of the sequences in their spicule proteins.
To solve this low-specific problem, researchers have developed an antibody test that detects a feature they believe is unique to sars-CoV-2 coronaviruses.
The researchers used part of the viral spicule called the receptor-binding domain (RBD), which binds to a particular protein in human cells to infect them: angiotensin-converting enzyme 2 (ACE2).
RBDs vary considerably between coronaviruses, and the RBD of SARS-CoV-2 has only a relevant similarity to the closely related SARS-CoV-1 virus, which caused the SARS outbreak of 2002-2004.
This type of antibody test can reveal whether someone has developed a strong immunity to the virus that causes COVID-19 with less chance that the test will give false-positive results.
The new test is 98% sensitive, meaning it would detect 98% of infections, and better yet, it’s 100% specific to antibodies produced to fight SARS-CoV-2 coronaviruses.
Neutralizing antibodies
While the immune system can generate a variety of antibodies that bind to different parts of a virus, only a few antibodies can neutralize it.
The researchers then went on to test whether their trial reflected the number of “neutralizing antibodies” in patients’ blood samples.
Working with colleagues at the UNC Gillings School of Global Public Health, the team found that the results of the new RBD-based test were strongly correlated with neutralizing antibody levels in the samples.
COVID-19 VACCINES
The speed of spread of the SARS-CoV-2 virus has made it a global pandemic. In addition, the lethality and sequelae it presents in infected patients have motivated the search for vaccines with diverse technologies, from traditional ones such as live attenuated virus and inactivated virus vaccines, to more modern technologies such as viral vector and nucleic acid vaccines, which are summarized in the following table:
| TYPE | FEATURES |
| Live attenuated virus vaccines | Composed of the attenuated SARS-CoV-2 virus itself, which has reduced its virulence and ability to reproduce. When confronted with this vaccine, the immune system learns to recognize and fight this weaker form of the virus, without the disease developing. |
| Inactivated virus vaccines | Contain dead or inactivated SARS-CoV-2 virus, complete or fragments thereof. When the immune system detects the dead virus or its fragments, it learns to recognize the SARS-CoV-2 virus and reacts by giving a defensive response. |
| Vaccines of virus subunits | They are made up of virus proteins. The protein is used in most laboratories is located on the surface of the SARS-CoV-2 virus, called “spike protein”. This protein is what allows the SARS-CoV-2 virus to bind to the surface of human cells and enter them, infecting them. The immune system recognizes this protein, and if it comes into contact with the virus, it prevents the entry of the virus into the cells preventing its replication and spread to other cells of the body. |
| Viral vector vaccines | This class of vaccines uses a virus that is harmless to our body as a messenger or vector, which has been genetically modified and has the ability to produce SARS-CoV-2 virus proteins (usually spike S protein) when it enters the body’s cells. The recognition of the immune system to this protein prevents the entry of the virus and therefore its replication and the establishment of the disease COVID-19. |
| DNA or RNA vaccines | The goal of this type of vaccine is to have the body itself directly produce a SARS-CoV-2 virus protein. The vaccine consists of plasmids or liposomes that contain a fragment of the coronavirus nucleic acid (DNA or mRNA) with the genetic information to make a specific protein (usually the spike S protein). Nucleic acid is inserted into human cells, producing copies of the virus protein, against which the immune system reacts. |
As we can see, most vaccines are focused on activating the immune system against the SARS-CoV-2 spike protein by producing neutralizing antibodies to block the binding of the virus to host cells and consequently prevent the development of COVID-19.
Therefore, a specific serological test that quantitatively measures neutralizing antibodies to SARS-CoV-2 in the plasma or serum of vaccinated patients is the ideal tool to evaluate the effectiveness of vaccination and track the duration of immunity generated by such vaccination.
MAXNAB ELISA KIT FOR NEUTRALIZING ANTIBODIES OF SARS-COV-2
There are several methods used to measure neutralizing antibodies to SARS-CoV-2, including:
- plate reduction neutralization test (PRNT)
- virus neutralization test (VNT)
- pseudovirus neutralization test (pVNT)
These methods are time-consuming, underperforming, and especially require live biological material or strict biosecurity containment measures for testing.
The SARS-CoV-2 MaxNAb neutralizing antibody test kit is fast and safe. It contains all the reagents needed to quantitatively measure the level of neutralizing SARS-CoV-2 antibodies.
In this kit, a sars-CoV-2 spike protein stable coats the wells of a microplate.
ACE2 His-labeled is bound to the spike protein coating according to the presence or absence of neutralizing antibodies contained in the sample.
The amount of ACE2 bound, which is proportional to the intensity of ACE2 inhibition, is then recognized by the neutralization detection complex containing anti-His antibodies.
The complex is revealed by an ELISA-like reaction with the development of a coloration whose absorbance is read in a spectrophotometer at a wavelength of 450nm.
The level of neutralizing antibodies is inversely proportional to the intensity of the measured optical density.
Detecting SARS-CoV-2 neutralizing antibodies in a quantitative, high-throughput manner can help:
(1) Identify when infected and/or vaccinated individuals have developed protective immunity against COVID-19 and how long neutralizing antibodies persist after infection.
(2) Accurately evaluate therapeutic antibodies against SARS-CoV-2
(3) Greatly benefit the development of effective vaccines.
In conclusion, the MaxNAb Kit ELISA for NEUTRALIZING ANTIBODIES of SARS-CoV-2 is one of the fastest, safest, and most specific method to measure the effectiveness of vaccination against COVID-19.
References:
• Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19
Nature Medicine volume 26, pages 453–455 (2020)
• Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum
Nature Immunology volume 22, pages 25–31 (2021)
• Antibody tests for COVID-19
Mayo Clinic
https://www.mayoclinic.org/es-es/tests-procedures/covid-19-antibody-testing/about/pac-20489696?p=1
• COVID-19 IgM / IgG antibodies by immunochromatographic assay (rapid test)
Medicine & Laboratory 2020; 24 (3)
• mRNA, proteins, adenovirus … how does each type of vaccine work and how is it different?
Government of Spain. Ministry of Health
https://www.vacunacovid.gob.es/arnm-proteinas-adenovirus-como-actua-y-en-que-se-diferencia-cada-tipo-de-vacuna
• The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients
Science Immunology 11 Jun 2020: Vol. 5, Issue 48, eabc8413
DOI: 10.1126 / sciimmunol.abc8413
• MaxNAb ELISA Kit for neutralizing antibodies to SARS-CoV-2
Protocol

Leave a Reply